Abstract
Background:
CAR-T cell therapy directed against the CD19 antigen is a breakthrough treatment for patients (pts) with relapsed/refractory (R/R) B-cell NHL. Despite impressive outcomes, not all pts respond and many that respond still relapse. Affordability and accessibility are further considerations that limit current commercial models of CAR-T products. Commercial CAR-T manufacturing is complex, time consuming, and expensive with a supply chain starting at the treating center with apheresis of mononuclear cells, cryopreservation, and shipping to and from a centralized third-party manufacturing site. We addressed these limitations in a Phase 1 clinical trial evaluating a first-in-human bispecific tandem CAR-T cell directed against both CD19 and CD20 (CAR-20.19-T) antigens for pts with R/R B-cell NHL. Through dual targeting we hope to improve response rates and durability of response while limiting antigen escape. We eliminated third party shipping logistics utilizing the CliniMACS Prodigy, a compact tabletop device that allows for automated manufacturing of CAR-T cells within a GMP compliant environment within the hospital. Most materials and reagents used to produce the CAR-T cell product were single-sourced from the device manufacturer.
Methods:
Phase 1 (NCT03019055), single center, dose escalation + expansion study to demonstrate feasibility and safety of locally manufactured second generation 41BB + CD3z CAR-20.19-T cells via the CliniMACS Prodigy. Feasibility was measured by ability to generate a target CAR-20.19-T cell dose for a minimum of 75% of subjects. Safety was assessed by the presence of dose limiting toxicities (DLTs) through 28 days post-infusion. Dose was escalated in a 3+3 fashion with a starting dose of 2.5 x 10^5 cells/kg, a target DLT rate <33%, and a goal treatment dose of 2.5 x 10^6 cells/kg. Adults with R/R Diffuse Large B-cell Lymphoma (DLBCL), Follicular Lymphoma (FL), Mantle Cell Lymphoma (MCL) or Chronic Lymphocytic Leukemia (CLL) were eligible.
CAR-T production was set for a 14-day manufacturing process. Day 8 in-process testing was performed to ensure quality and suitability of CAR-T cells for a potential fresh infusion. On Day 10, pts eligible for a fresh CAR-T infusion initiated lymphodepletion (LDP) chemotherapy with fludarabine 30 mg/m2 x 3 days and cyclophosphamide 500 mg/m2 x 1 day, and cells were administered after harvest on Day 14. Pts ineligible for fresh infusion received cryopreserved product and LDP was delayed accordingly.
Results:
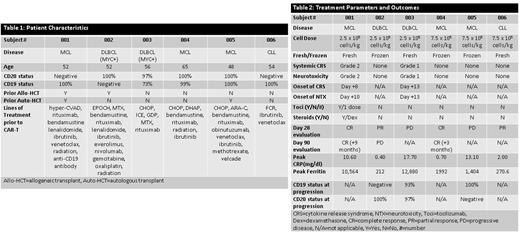
6 pts have been enrolled and treated with CAR-20.19-T cells: 3 pts at 2.5 x 10^5 cells/kg and 3 pts at 7.5 x 10^5 cells/kg. Median age was 53 years (48-62). Underlying disease was MCL in 3 pts, DLBCL in 2 pts, and CLL in 1 patient. Baseline data and prior treatments are listed in Table 1. CAR-T production was successful in all runs and all pts received their target dose. Three pts received fresh CAR-T cells and 3 pts received CAR-T cells after cryopreservation. To date there are no DLTs to report. No cases of Grade 3/4 cytokine release syndrome (CRS) or neurotoxicity (NTX) were observed. One patient had Grade 2 CRS and Grade 2 NTX requiring intervention. The other had self-limited Grade 1 CRS and Grade 1 NTX. Median time to development of CRS was Day +11 post-infusion. All pts had neutrophil recovery (ANC>0.5 K/µL) by Day 28. Response at Day 28 (Table 2) is as follows: 2/6 pts achieved a complete response (CR), 2/6 achieved a partial response (PR), and 2/6 had progressive disease (PD). One subject with a PR subsequently progressed at Day 90. The 3 pts who did progress all underwent a repeat biopsy, and all retained either CD19 or CD20 positivity. Pts are currently being enrolled at the target dose (2.5 x 10^6 cells/kg) and updated results will be provided at ASH.
Conclusions:
Dual targeted anti-CD19 and anti-CD20 CAR-T cells were successfully produced for all pts demonstrating the feasibility of a point-of-care manufacturing process via the CliniMACS Prodigy device. With no DLTs or Grade 3-4 CRS or NTX to report, and 2/6 heavily pre-treated pts remaining in CR at 3 and 9 months respectively our approach represents a feasible and promising alternative to existing CAR-T models and costs. Down-regulation of both target antigens was not identified in any patient following CAR-T infusion, and in-process studies suggest that a shorter manufacturing timeline is appropriate for future trials (10 days).
Shah:Juno Pharmaceuticals: Honoraria; Lentigen Technology: Research Funding; Oncosec: Equity Ownership; Miltenyi: Other: Travel funding, Research Funding; Geron: Equity Ownership; Exelexis: Equity Ownership. Zhu:Lentigen Technology Inc., A Miltenyi Biotec Company: Research Funding. Schneider:Lentigen Technology Inc., A Miltenyi Biotec Company: Employment. Krueger:Lentigen Technology Inc., A Miltenyi Biotec Company: Employment. Worden:Lentigen Technology Inc., A Miltenyi Biotec Company: Employment. Hamadani:Sanofi Genzyme: Research Funding, Speakers Bureau; Merck: Research Funding; Janssen: Consultancy; MedImmune: Consultancy, Research Funding; Cellerant: Consultancy; Celgene Corporation: Consultancy; Takeda: Research Funding; Ostuka: Research Funding; ADC Therapeutics: Research Funding. Johnson:Miltenyi: Research Funding. Dropulic:Lentigen, A Miltenyi Biotec company: Employment. Orentas:Lentigen Technology Inc., A Miltenyi Biotec Company: Other: Prior Employment. Hari:Takeda: Consultancy, Honoraria, Research Funding; Janssen: Honoraria; Kite Pharma: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Spectrum: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Amgen Inc.: Research Funding; Sanofi: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal